The effect created by the reactivity in one part of the molecule due to the electron attraction or repulsion is termed the electronic effect. During this action, electrons are contributed for bonding between the atoms. There are four types of electronic effects: Inductive effect, Mesomeric Effect, Hyperconjugal effect, and Electrometric effect.
- Inductive effect: Inductive effect occurs when dissimilar atoms share a bonding electron causing an unequal transmission of charge through the chain of atoms. The more electronegative atom pulls the electrons towards itself leading to a covalent bond polarization. The polarity produced in the molecule by sigma bond interaction and is permanent.
- Mesomeric Effect: Mesomeric effect happens due to the permanent polarization of a molecule when substituent group or functional group interacts with chemical compounds through pi- bond electron transfer. In other words, the mesomeric effect occurs when pi- electrons move away or towards the conjugated p- orbital system.
- Hyperconjugal effect: Hyperconjugation explains the stability of alkyl radicals occurring due to delocalization of the sigma electrons of the carbon-hydrogen bond of an alkyl group and permanently attaching to an atom with an unshared p-orbital.
- Electrometric effect: Electromeric Effect is a temporary and reversible effect that happens due to the intramolecular shifting of electrons from a pi-bond to another atom during an introduction of a reagent.
During the inductive effect the atoms pair electrons to form a covalent single bond. These electrons can be contributed from similar or dissimilar atoms. In the case of dissimilar atoms, there is a difference of electronegativity resulting in a polarized covalent bond where the bonded electrons are shifted to the more electronegative atom. This inductive effect causes an unequal transmission of charge through the chain of atoms. This electronic effect occurs in a sigma bond and is a permanent effect making the greater electronegative atom partial negative charged while the contiguous chain of atoms of the molecule becomes partial positive charged.
Whether the moiety of the molecule attracts from or donates electrons towards the chain of atoms, the inductive effect is either negative or positive effects (-I or +I effect). When an electronegative atom is attached to a chain of atoms, which are mostly carbons, electrons are paired up and a positive charge is relayed towards the other atoms in the chain causing an electron-withdrawing effect or in other words, called the negative inductive effect. All functional groups except alkyl groups, viz., ester-group, amide- group, hydroxyl- group, ketone- group, amine- group, amino- group, aldehyde- group, an ether- group cause a negative inductive effect. Comparing to a hydrogen atom, an alkyl group possesses lesser electronegativity and they tend to push the bonding electron, creating a relay of positive charge through this functional group. The tendency of donating electrons makes this group show a positive inductive effect.
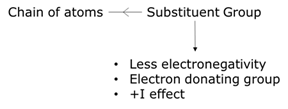
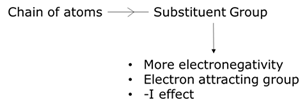
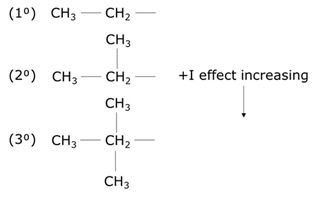
Factors influencing positive inductive effect
- Induced polarity reduces with greater the distance between the electron-withdrawing atom and other atoms of the chain system.

- The more carbon atoms attached to an alkyl group, the greater is the positive inductive effect for which methyl is having a lesser positive inductive effect than ethyl.
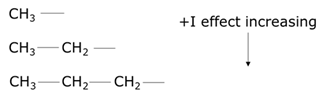
- The greater the degree of the alkyl group, the greater is the positive inductive effect. Therefore, a tertiary alkyl has a greater electron-donating effect than a secondary alkyl.
Generally, alkyl groups are positive inductive affecting groups with the few exceptions:
a. Alkyl group are supposedly donating electrons, making it an electron-donating group except for organometallics like Grignard reagents where the metal atom donates electrons converting the alkyl group to be electronegative.

b. Within the chain of alkyl groups, the more electronegative group will be the group with a greater S-character. Greater s-character means greater will be electronegativity due to more contribution of the sigma type bond in a hybridization. Therefore, greater electron-withdrawing potency of sp2 bond has more negative inductive effect than sp3 bond.
Factors influencing negative inductive effect
- In other functional groups, other than the alkyl group, there is an electron-withdrawing atom (EWA) that makes the functional group causes a negative inductive effect. The strength of the negative inductive effect of the functional group depends on the location of the electron-withdrawing atom from the linking carbon atom (C1). Farther the location of EWA from the linking carbon atom (C1), weaker becomes the negative inductive effect of the functional group.

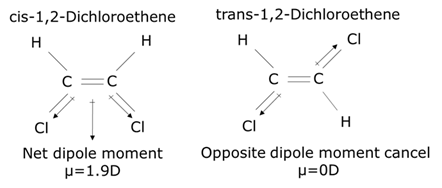
- Dipole moment occurs due to the difference in electronegativity of ionic or covalent bonded atoms. Bond dipole moment is a vector quantity that measures the magnitude and direction of the polarity of the chemical bond in the system. Being a vector quantity, the dipole moment can be zero as the two bond dipoles cancel each other as they are opposite and equal, otherwise, the polarity adds up to give a dipole moment. Dipole moment directly influences the negative inductive effect. For example, a cis-1,2- Dichloroethene has a more negative inductive effect than a trans-1,2- Dichloroethene.